Solubility is a critical physical property of organic compounds in drug development, e.g., availability, distribution, metabolism, excretion and toxicity (ADMET) 1,2, protein engineering 3,4,5.. solubility in 10 organic solvents at 298.15, 323.15, and 373.15 K, and partially at pressures of up to 14 MPa. Literature data on the solubility of hydrogen in liquids has
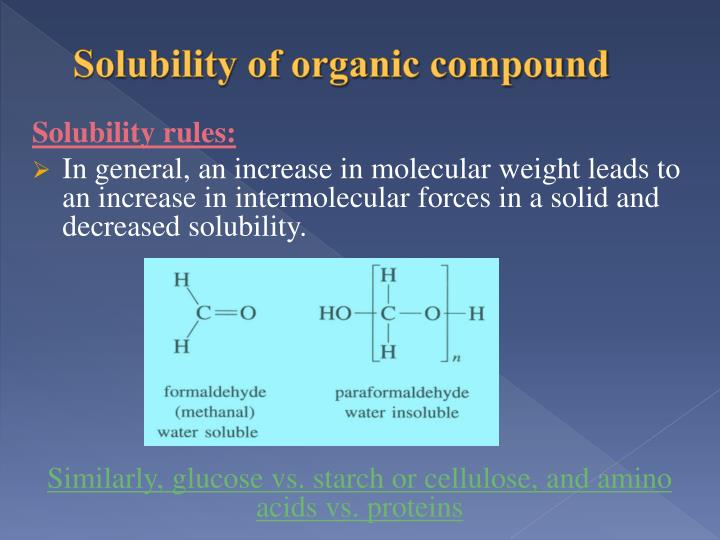
PPT Solubility of organic compound organic chemistry II lab PowerPoint Presentation ID3105359

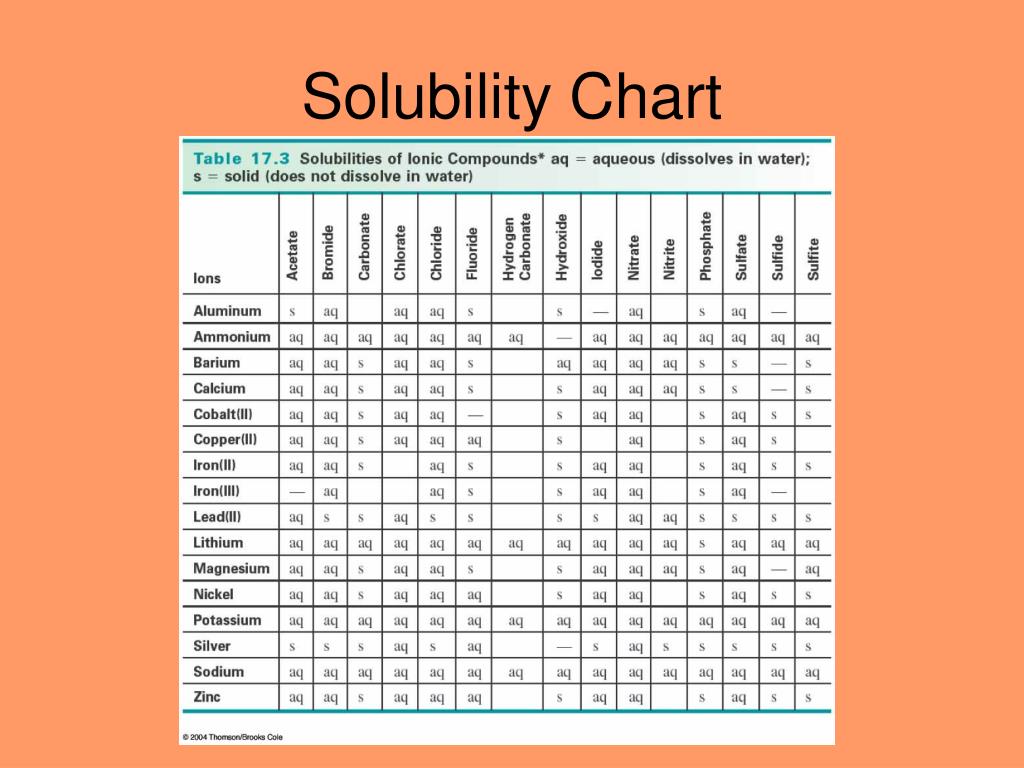
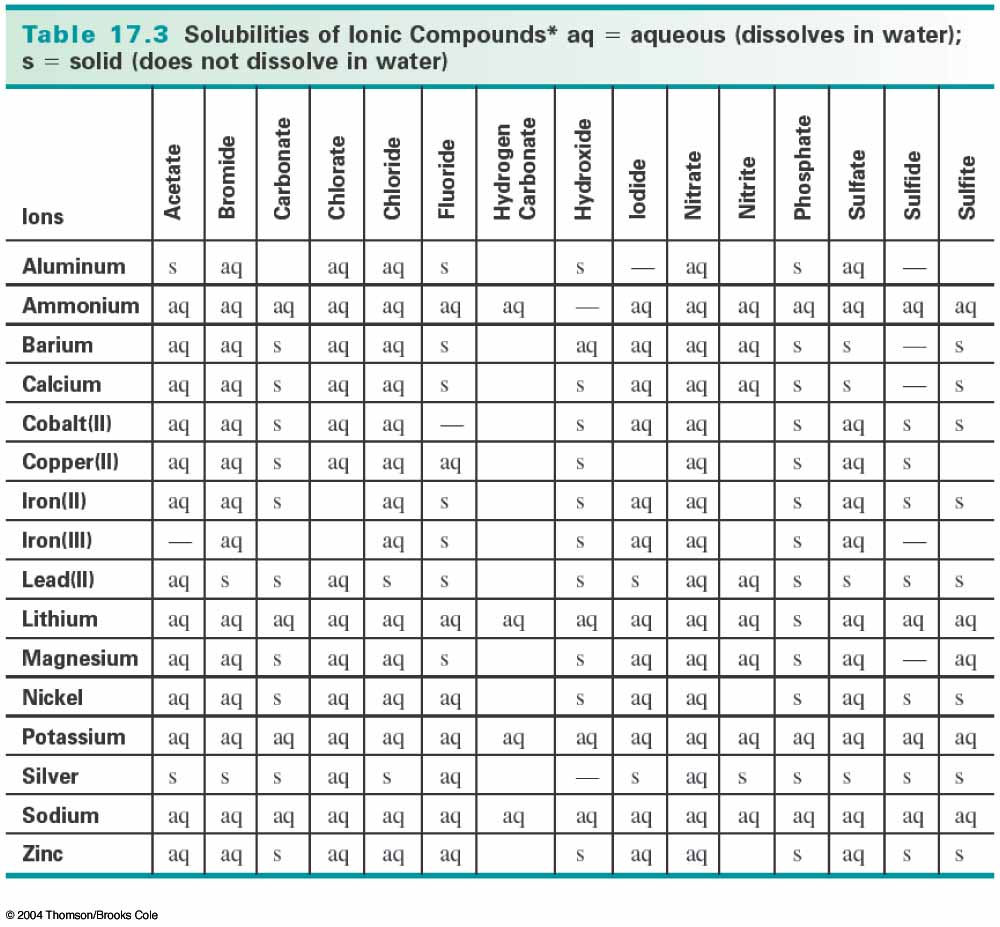
Solubility In Water Chart

Summary of Hydrogen Solubility for Adsorbates at 303.15 K Download Scientific Diagram
Solubility Rules Flowchart Chart Chemistry
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free download ID6682844
Question 5c03c Socratic

Solubility of HydrogenBonded Molecules, Chemistry Lecture Sabaq.pk YouTube

Solubility of Organic Compounds Chemistry Steps

Solubility of polyester sheet in organic solvents, water, alkaline, and... Download Scientific
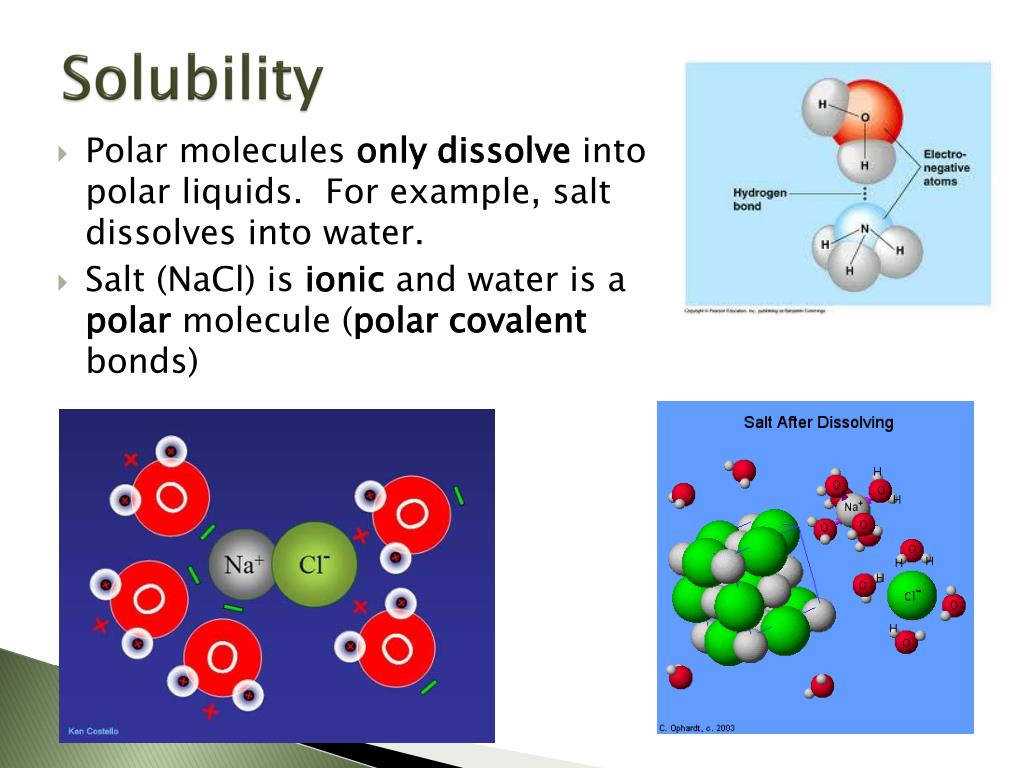
PPT Solubility PowerPoint Presentation, free download ID2493612
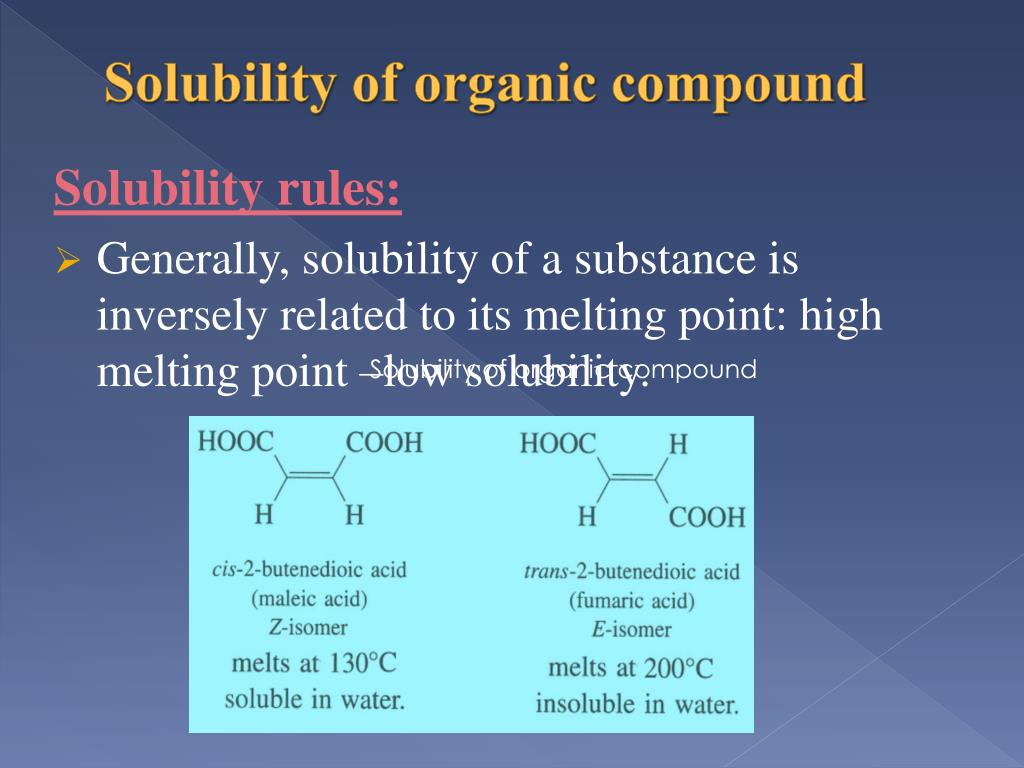
PPT Solubility of organic compound organic chemistry II lab PowerPoint Presentation ID3105359

Solubility Rules Pathways to Chemistry
Solubility of hydrogen in H 2 O in the course of decreasing temperature... Download Scientific
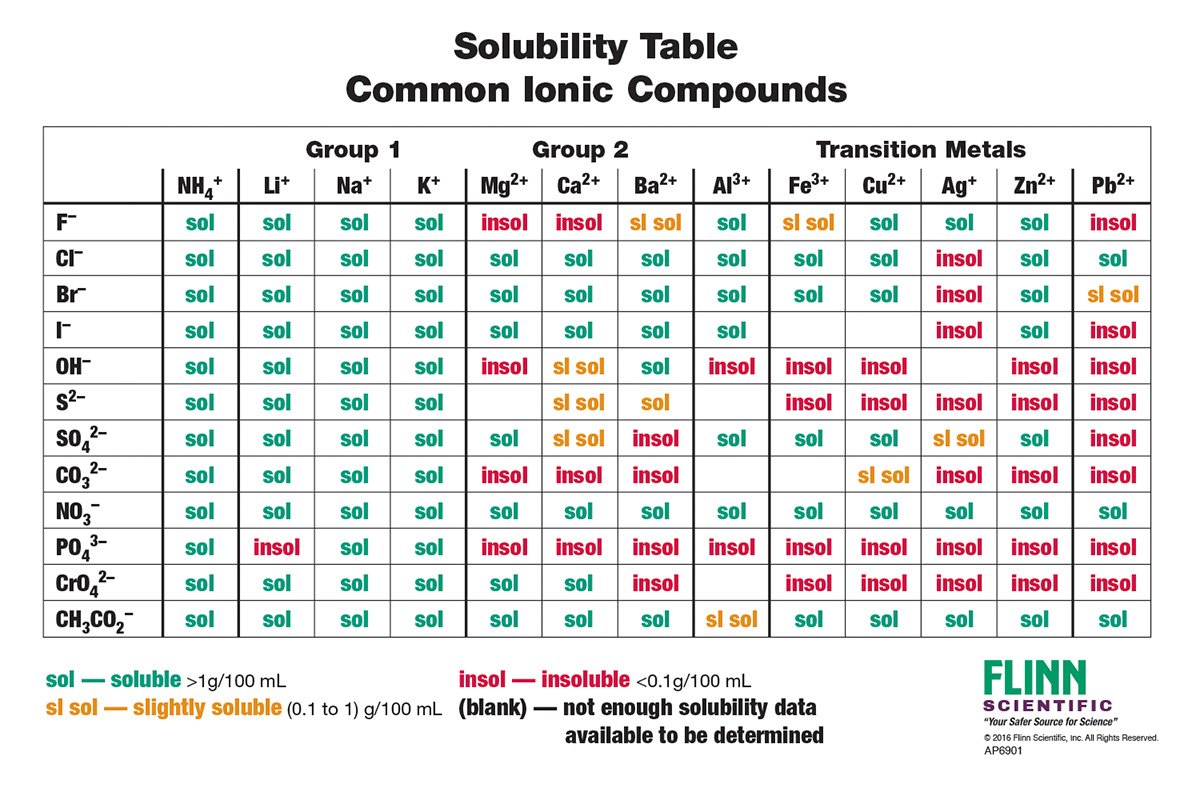
Solubility Rules Charts for Chemistry
PPT Solubility PowerPoint Presentation, free download ID6260427

Solubility and gelation properties of BHPBIA in various organic solvents. a Download Table

Solubility of Organic Compounds Chemistry Steps
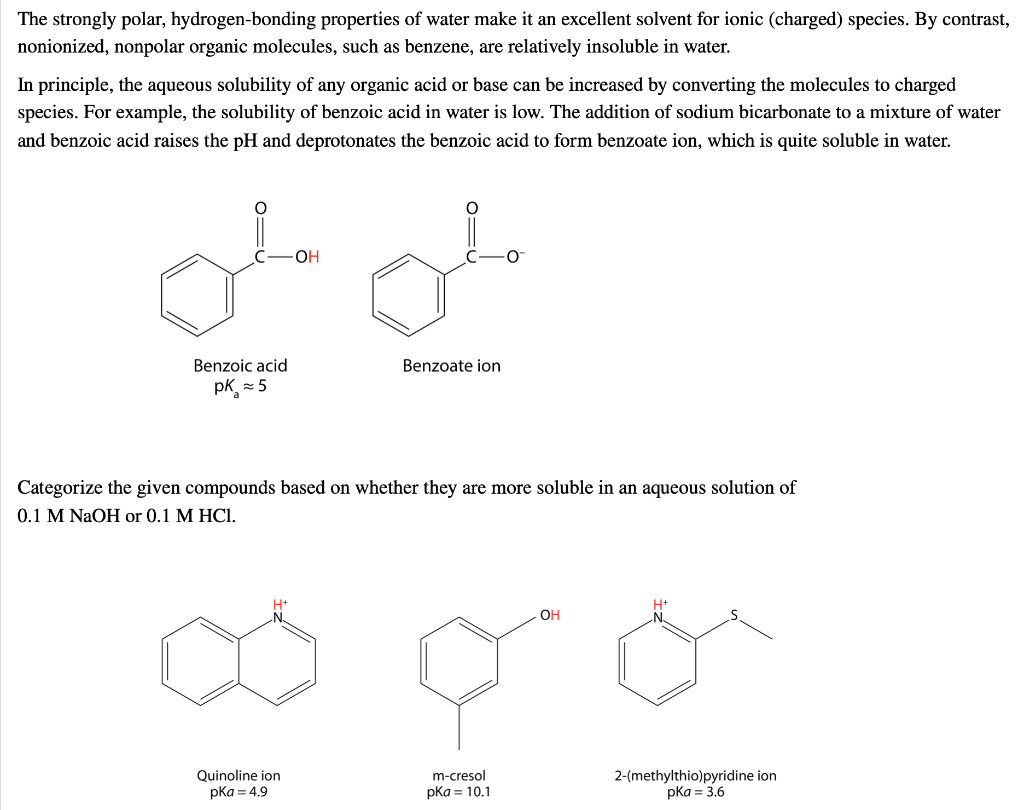
Solved The strongly polar, hydrogenbonding properties of

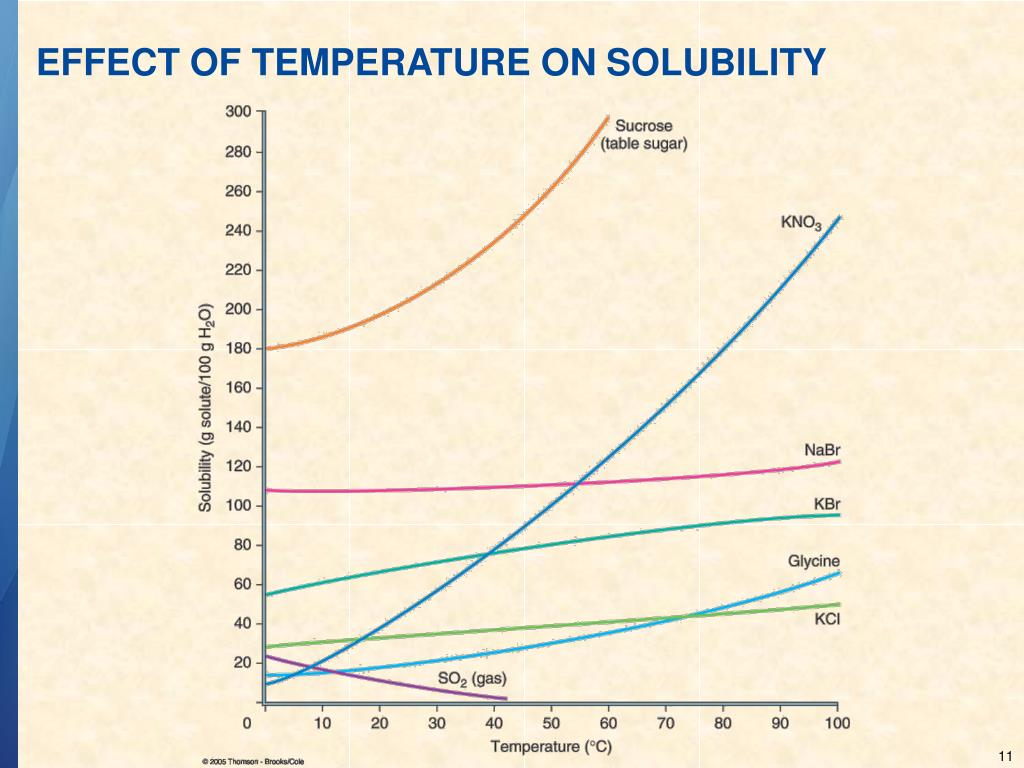
13.3 Factors Affecting Solubility Chemistry LibreTexts

Solubility Rules
The solvatochromicity of established solvatochromic UV/Vis probes, which appear to be sensitive to the so-called hydrogen bond donor (HBD) property of the solvent, is analysed using the hydroxyl group density of alcoholic solvents D HBD as a physical parameter in comparison to the pKa, the chemical benchmark for acidity. Reichardt's dye B30, Kosowers Z-indicator 1-ethyl-4-(methoxycarbonyl.. The solubility of hydrogen in the organic solvents is generally very small and therefore Henry's constant is a valuable descriptor of the solubility [20]. The binary systems of hydrogen with n.